Your Which material undergoes radioactive decay images are available. Which material undergoes radioactive decay are a topic that is being searched for and liked by netizens today. You can Download the Which material undergoes radioactive decay files here. Get all free photos.
If you’re searching for which material undergoes radioactive decay pictures information linked to the which material undergoes radioactive decay interest, you have pay a visit to the right site. Our site frequently gives you suggestions for downloading the maximum quality video and picture content, please kindly surf and locate more enlightening video articles and graphics that fit your interests.
Which Material Undergoes Radioactive Decay. A radioactive sample is undergoing α decay. Three of the most common types of decay are alpha decay beta decay and gamma decay all of which involve emitting one or more particles or photons. Then the effective decay constant lambdaeff is. At any time t1 its activity is A and another time t2.

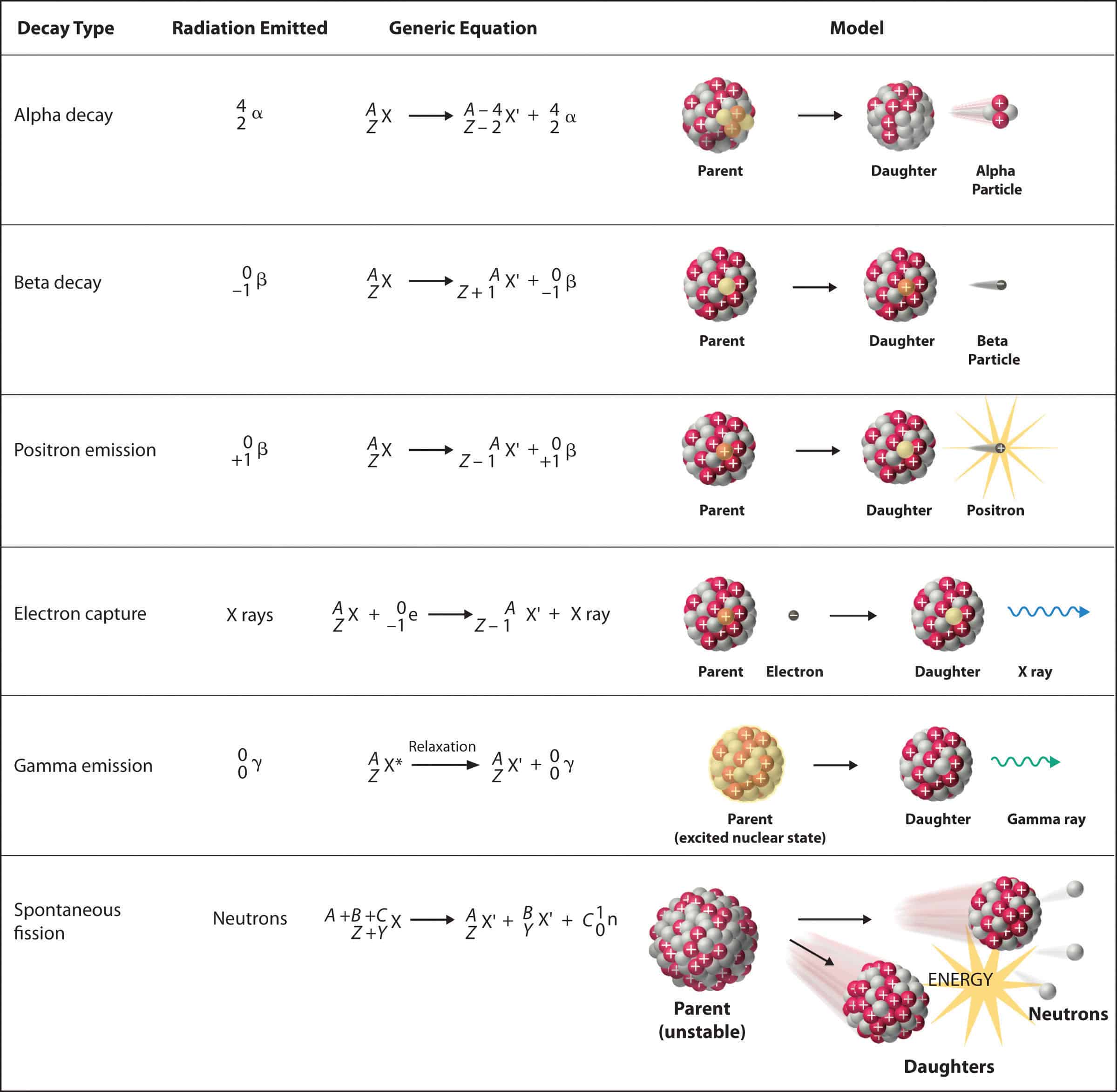
A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda. How is radioactive decay used to date sedimentary rocks. At any time t1 its activity is A and another time t2. The radiation produced during radioactive decay is such that the daughter nuclide lies closer to the band of stability than the parent nuclide so the location of a nuclide relative to the band of stability can serve as a guide to the kind of decay it will undergo. The radioactive decay law states that The probability per unit time that a nucleus will decay is a constant independent of time. Then the effective decay constant lambdaeff is equal to nlambda.
What is the process of nuclear change in an atom of radioactive material.
Then the effective decay constant lambdaeff is. Then the effective decay constant lambdaeff is. What is the value of n. The radioactive decay law states that The probability per unit time that a nucleus will decay is a constant independent of time. All elements that have turned into a new element D. What is radioactive decay.
 Source: radiation-dosimetry.org
Source: radiation-dosimetry.org
All elements that have a half-life B. In radioactive decay an atom will lose protons and therefore forms new elements. The radiation produced during radioactive decay is such that the daughter nuclide lies closer to the band of stability than the parent nuclide so the location of a nuclide relative to the band of stability can serve as a guide to the kind of decay it will undergo. Ymlantigua21 A material containing unstable nuclei is considered radioactive. A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda.
 Source: toppr.com
Source: toppr.com
All elements that contain atoms with protons Subjects. Radioactive decay also known as nuclear decay or radioactivity is a random process by which an unstable atomic nucleus loses its energy by emission of radiation or particle. This would be C because radioactive decay is when atoms and protons decide to slowly decay or separate from the atom. Then the effective decay constant lambdaeff is equal to nlambda. The amounts of carbon-14 and nitrogen.
 Source: byjus.com
Source: byjus.com
What is the value of n. All elements that contain atoms with protons C. All elements that contain atoms with protons Subjects. Then the effective decay constant lambdaeff is equal to nlambda. What is radioactive decay.

Three of the most common types of decay are alpha decay beta decay and gamma decay all of which involve emitting one or more particles or photons. How is radioactive decay used to date sedimentary rocks. All elements that have a half-life B. Radioactive decay also known as nuclear decay or radioactivity is a random process by which an unstable atomic nucleus loses its energy by emission of radiation or particle. A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda.
 Source: epa.gov
Source: epa.gov
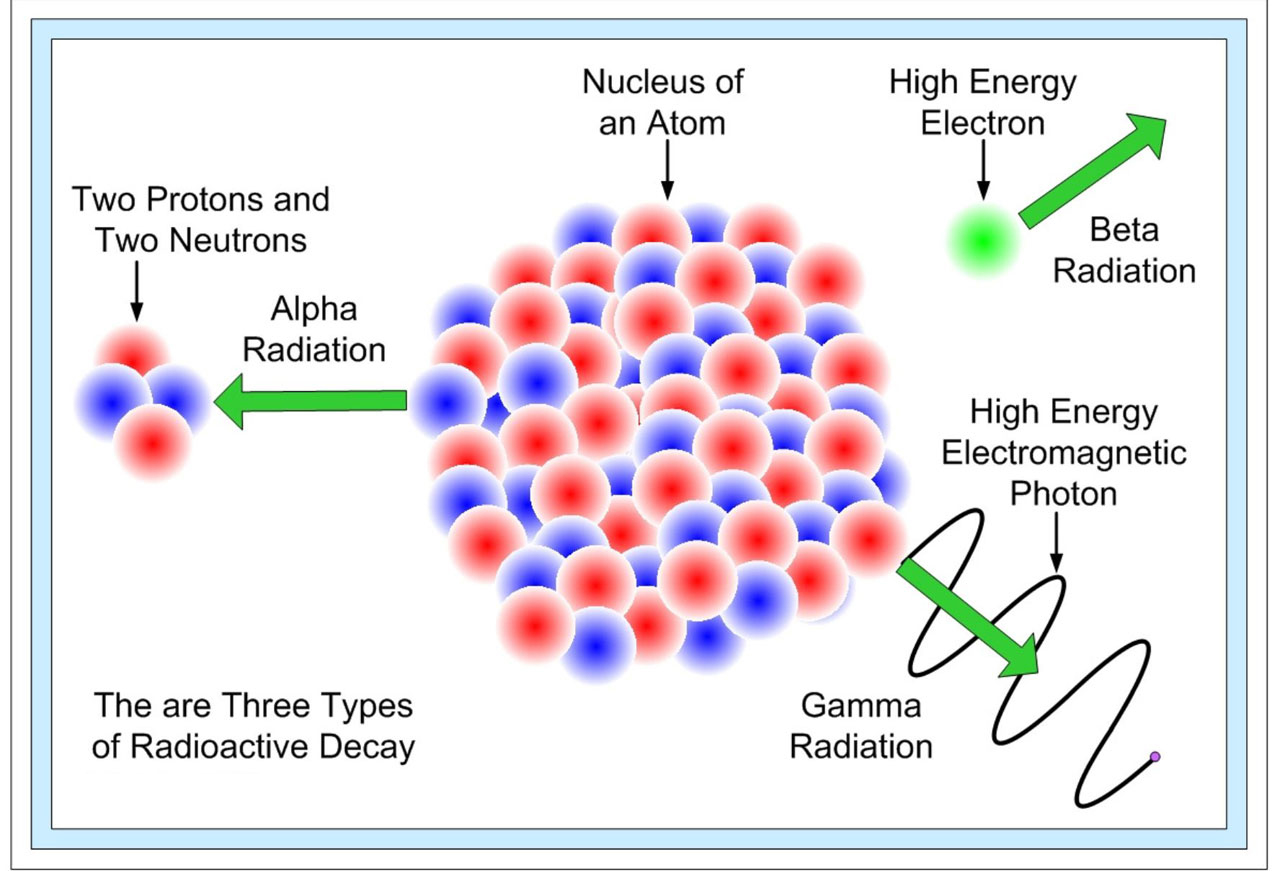
Then the effective decay constant lambdaeff is. The radiation produced during radioactive decay is such that the daughter nuclide lies closer to the band of stability than the parent nuclide so the location of a nuclide relative to the band of stability can serve as a guide to the kind of decay it will undergo. In radioactive decay an atom will lose protons and therefore forms new elements. How does an atom change when it undergoes radioactive decay. A radioactive sample is undergoing α decay.
 Source: geigercounter.org
Source: geigercounter.org
Then the effective decay constant lambdaeff is equal to nlambda. The amounts of potassium and argon in sedimentary rocks are measured. How does an atom change when it undergoes radioactive decay. What is radioactive decay. A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda.
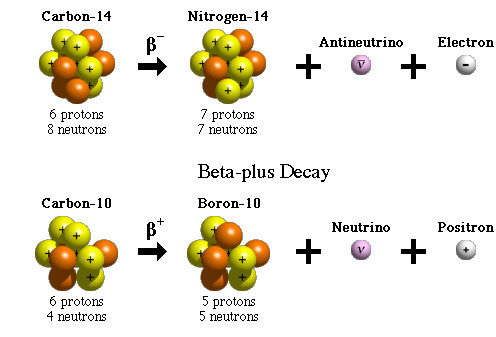 Source: scienceabc.com
Source: scienceabc.com
At any time t1 its activity is A and another time t2. 2 question HURRY Which material undergoes radioactive decay. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay beta decay and gamma decay all of which involve emitting one or more particles or photons. A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda.
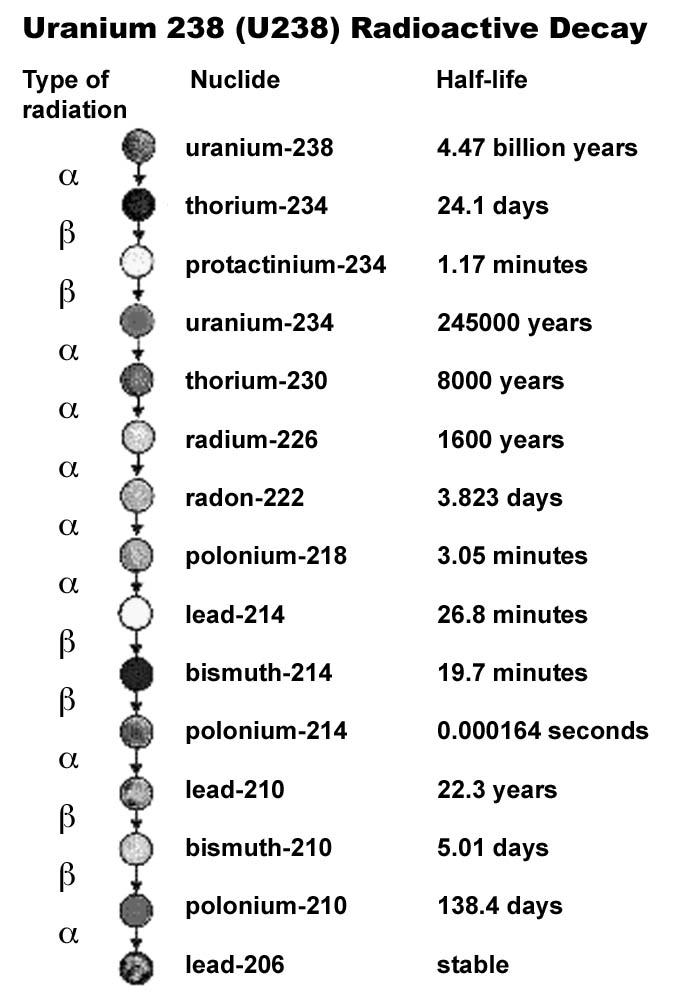 Source: imagine.gsfc.nasa.gov
Source: imagine.gsfc.nasa.gov
Then the effective decay constant lambdaeff is equal to nlambda. This would be C because radioactive decay is when atoms and protons decide to slowly decay or separate from the atom. According to the radioactive decay law when a radioactive material undergoes either 𝛼 or β or ℽ decay the number of nuclei undergoing the decay per unit time is proportional to the total number of nuclei in the given sample material. In radioactive decay an atom will lose protons and therefore forms new elements. A material containing unstable nuclei is considered radioactive.
 Source: energyeducation.ca
Source: energyeducation.ca
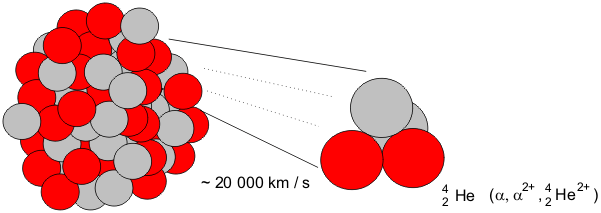
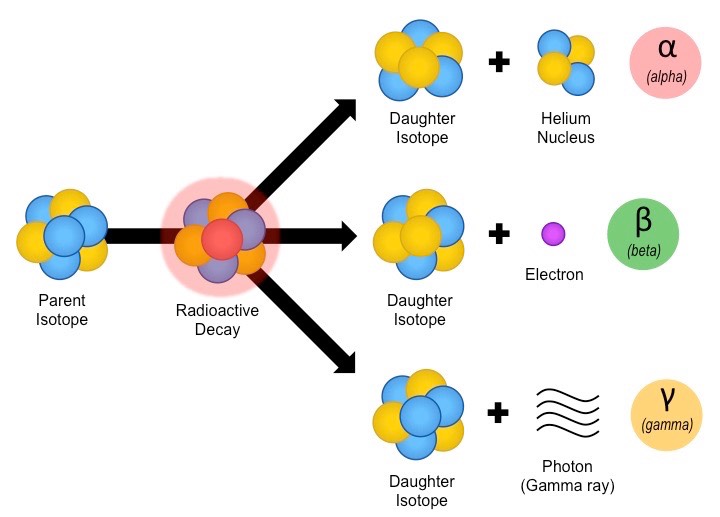
In alpha decay an alpha particle a helium nucleus is emitted from the radioactive atom and the atom therefore loses 2 protons and becomes a new element. Correct answer - HURRY Which material undergoes radioactive decay. All elements that contain atoms with protons C. A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda. Ymlantigua21 A material containing unstable nuclei is considered radioactive.
 Source: sciencenotes.org
Source: sciencenotes.org
The process of nuclear change in an atom of radioactive material is called nuclear decay. According to the radioactive decay law when a radioactive material undergoes either 𝛼 or β or ℽ decay the number of nuclei undergoing the decay per unit time is proportional to the total number of nuclei in the given sample material. How is radioactive decay used to date sedimentary rocks. 2 question HURRY Which material undergoes radioactive decay. Three of the most common types of decay are alpha decay beta decay and gamma decay all of which involve emitting one or more particles or photons.
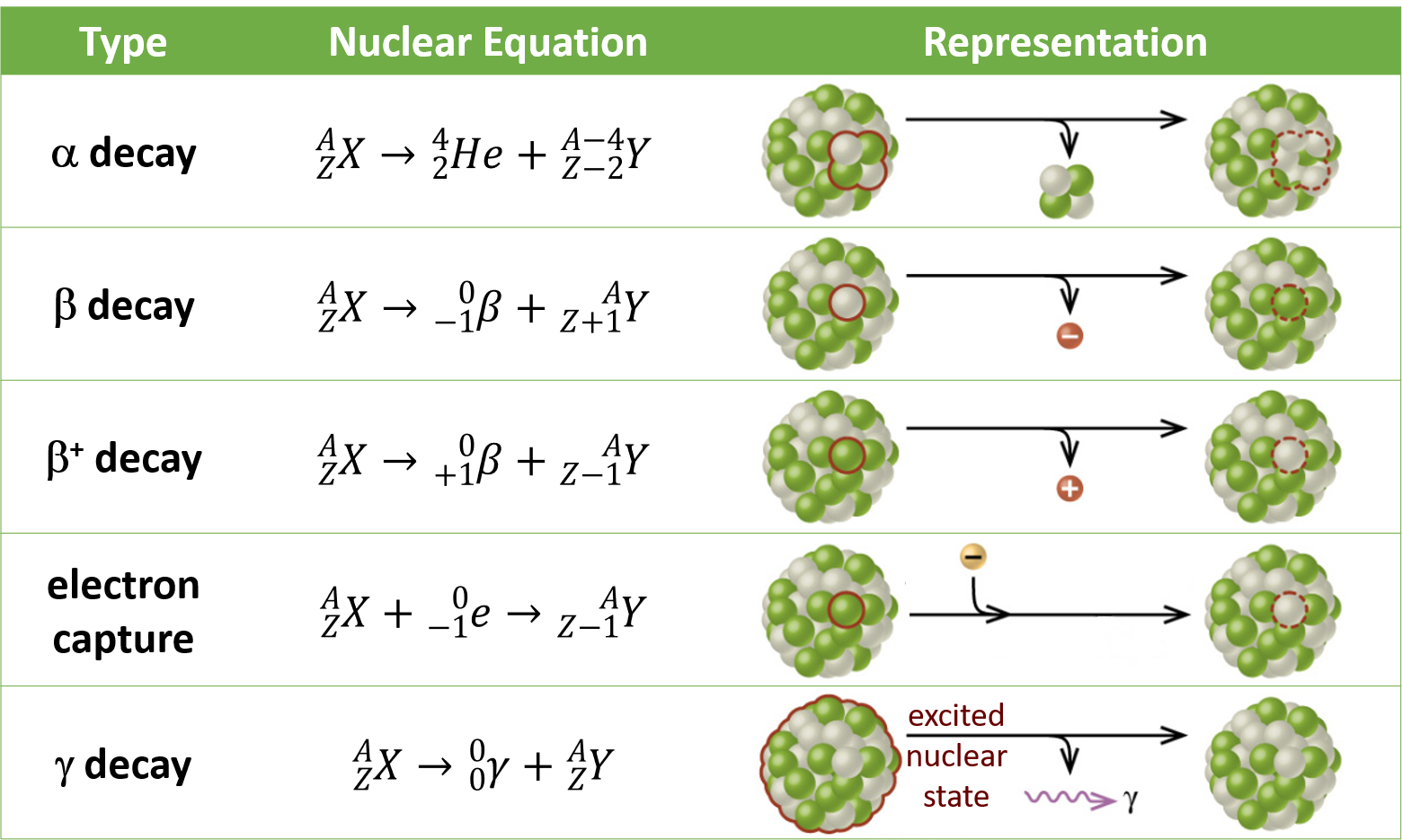 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
Radioactive decay also known as nuclear decay or radioactivity is a random process by which an unstable atomic nucleus loses its energy by emission of radiation or particle. A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda. Three of the most common types of decay are alpha decay beta decay and gamma decay all of which involve emitting one or more particles or photons. In radioactive decay an atom will lose protons and therefore forms new elements. This would be C because radioactive decay is when atoms and protons decide to slowly decay or separate from the atom.
 Source: cnx.org
Source: cnx.org
A material containing unstable nuclei is considered radioactive. A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda. All elements that have a half-life B. 2 question HURRY Which material undergoes radioactive decay. The amounts of carbon-14 and nitrogen.

All elements that have a half-life B. What is radioactive decay. The radioactive decay law states that The probability per unit time that a nucleus will decay is a constant independent of time. The radiation produced during radioactive decay is such that the daughter nuclide lies closer to the band of stability than the parent nuclide so the location of a nuclide relative to the band of stability can serve as a guide to the kind of decay it will undergo. What is the process of nuclear change in an atom of radioactive material.
 Source: cnx.org
Source: cnx.org
A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda. A radioactive sample is undergoing α decay. The amounts of carbon-14 and nitrogen. A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda. In radioactive decay an atom will lose protons and therefore forms new elements.
 Source: ausearthed.blogspot.com
Source: ausearthed.blogspot.com
All elements that contain atoms with protons Subjects. A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda. The radioactive decay law states that The probability per unit time that a nucleus will decay is a constant independent of time. The amounts of potassium and argon in sedimentary rocks are measured. Radioactive decay also known as nuclear decay or radioactivity is a random process by which an unstable atomic nucleus loses its energy by emission of radiation or particle.
 Source: radiation-dosimetry.org
Source: radiation-dosimetry.org
All elements that contain atoms with protons C. Three of the most common types of decay are alpha decay beta decay and gamma decay all of which involve emitting one or more particles or photons. What is the value of n. Then the effective decay constant lambdaeff is equal to nlambda. How does an atom change when it undergoes radioactive decay.
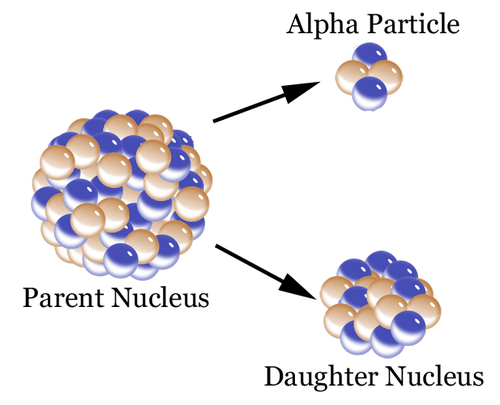
In alpha decay an alpha particle a helium nucleus is emitted from the radioactive atom and the atom therefore loses 2 protons and becomes a new element. A certain radioactive material can undergo three different types of decay each with a different decay constant lambda 2lambda and 3lambda. All elements that have a half-life B. According to the radioactive decay law when a radioactive material undergoes either 𝛼 or β or ℽ decay the number of nuclei undergoing the decay per unit time is proportional to the total number of nuclei in the given sample material. A material containing unstable nuclei is considered radioactive.
 Source: study.com
Source: study.com
Three of the most common types of decay are alpha decay beta decay and gamma decay all of which involve emitting one or more particles or photons. What is the process of nuclear change in an atom of radioactive material. According to the radioactive decay law when a radioactive material undergoes either 𝛼 or β or ℽ decay the number of nuclei undergoing the decay per unit time is proportional to the total number of nuclei in the given sample material. What is the value of n. Radioactive decay also known as nuclear decay or radioactivity is a random process by which an unstable atomic nucleus loses its energy by emission of radiation or particle.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title which material undergoes radioactive decay by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






